Hello, there! Dr. Rukundo is back. In today’s blog, we’re diving into the topic of identity and class – but not in the way you might think. While this might sound like something related to DEI or social segregation, we’re talking about the chemical and/or biological identity of materials that we work with every day.... Continue Reading →
All Things Snacks! Advancing Cereal and Snack Production with NIR Spectroscopy for Quality Control and Efficiency
Hello! Dr. Rukundo here, NIR Applications Specialist at BUCHI Corporation. If you have ever found yourself craving that extra bite of a sugary snack or reaching for another cereal pack, you might be wondering what draws us to these treats. In Sapiens: A Brief History of Humankind, the author argues that our evolutionary craving for... Continue Reading →
The Role of Near Infrared Spectroscopy (NIR) in Fertilizer Analysis: A Critical Element for Sustainable Agriculture and Food Security
Greetings, reader, Dr. Rukundo here! I am the NIR Applications Specialist at BUCHI Corporation. It is easy to assume humans raise plants, but have you ever thought that maybe plants are actually raising us? I know I have. Consider this: we need plants to survive, but plants do not necessarily need us. They were here... Continue Reading →
Boost Yogurt Quality Using NIR Spectroscopy
Near-Infrared (NIR) Spectroscopy can revolutionize yogurt production by enabling real-time, accurate, and efficient fermentation process monitoring. By leveraging this technology, yogurt producers can achieve consistent product quality, minimize waste, and improve overall production efficiency. Yogurt, or yoghurt – if you are British – has been a part of the human diet for millennia. History has... Continue Reading →
Addressing labor shortages, workplace safety, and slow QC feedback loops with NIR.
BUCHI NIR Specialist Jason Corbiere met up with BUCHI Market Manager Ryanne Palermo to discuss how benchtop or in-line NIR can address three major areas of concern for QC labs: labor shortages, workplace safety, and slow QC feedback loops. Watch the video discussion here or read the transcript below to learn more. https://youtu.be/xfNYV98EnIg Transcript RP:... Continue Reading →
Turn Industrial Waste into Profit with Real-Time Alternative Fuel Monitoring
Is your company looking for new revenue streams or to meet corporate green initiatives? By improving efficiency and utilization of low-carbon fuels in industrial waste, companies can secure energy needed for their business, all while reducing their carbon footprint or even achieving a net-zero carbon emission. BUCHI NIR Process Specialist Ryan Vautherot joined us... Continue Reading →
How much does a NIR system cost (and why)?
NIR is invisible to the naked eye, but sometimes the cost of near-infrared (NIR) spectroscopy systems may feel a little too transparent, too. To be fair, the answer to “How much does a NIR system cost?” really is “it depends.” Materials, hardware features, software, and licenses will all play into the final cost. In this... Continue Reading →
Three ways to consider ROI with NIR
Labs across the country are constantly asked to do more with less… fewer people, less time, and less budget. The value in a capital investment shows up where the rubber meets the road—in lab productivity and company profitability. Near-infrared (NIR) spectroscopy is a well-known technique that delivers fast results for standard quality parameters, helping break... Continue Reading →
Clean up your QC! Monitoring Soap with NIR
Did you celebrate National Shower with a Friend Day last Month? Before you start to think that this blog has gotten NSFW (not safe for work), let us explain. The cheeky National Shower with a Friend Day was born out of a marketing strategy by a water filtration company in 2014. The goal: educate people... Continue Reading →
Transcend your calibration transfer woes in 10 steps (with Transpec)
A New Year means new beginnings! Heading into 2022 we’ve added some new members to our group: Isaac Rukundo and Kristen Frano are two product specialists who have recently joined the BUCHI NIR team! They contributed to the content of this post. We’re now amid that post-holiday trudge! Were you good enough last year for... Continue Reading →
Choose your Adventure: Taking NIR from the bench to production
This editorial on NIR calibration transfer from benchtop to production was submitted by Mark Sullivan, a Senior NIR Applications Specialist for BUCHI North America. Not long after many new users become accustomed to the speed and convenience of laboratory NIR over conventional techniques, it dawns on them that this technology is inherently suited for use... Continue Reading →
NIR as a tool for real-time quality determination of Distiller’s Dried Grains
In a prior blog, we shared how NIR online sensors could be used to optimize biofuel processes, starting at the farm, through fuel processing, and into feed applications. In this post we take a closer look at how NIR can be used as a measurement tool for a valuable co-product of the biofuel industry: distiller’s dried grains with solubles (DDGS). Most ethanol plants in the United States are dry-grind facilities, which use starch... Continue Reading →
Tales from the Lab: NIR vs. Dumas for Carbon and Nitrogen Determination
This blog was contributed by Dr. Mark Sullivan, Technical and Application Specialist at BUCHI Corporation. The head of a well-known agricultural testing laboratory recently approached me with an unusual request. This laboratory performs 10’s of thousands of plant tissue analyses annually using the Dumas combustion technique for total nitrogen and carbon (AOAC Official Method 972.43).... Continue Reading →
From the Field to Fuel to Food: On-line Process Control of Grain, Biofuel and Co-Products
Summer is coming to a close, and the once plentiful corn stands that dotted country roads and even well-traversed city highways are beginning to dwindle down in number (replaced by donut stands, thankfully). Despite countless summer BBQs tugging on the supply chain at peak season, the vast landscape of cornfields in the Midwest (and beyond)... Continue Reading →
Primary Method Feature: Extraction (Part 1: Extraction Foundations)
Another blog not about NIR? Near-infrared (NIR) spectroscopy is a secondary method. That means that NIR doesn't measure things like fat, protein, moisture, ash, %-polymerization or anything else directly. Instead, we use an acceptable primary method to train our NIR to make those measurements. More details on NIR calibration can be found in this earlier... Continue Reading →
NIR vs. Kjeldahl
Protein is a critical parameter across the food, beverage, and feed industries. However, the best solution for obtaining protein varies across the production cycle. In this blog, we propose Kjeldahl and near-infrared (NIR) spectroscopy methods for protein determination, with arguments for which technology is the best fit based on factors such as: Application scope Variation... Continue Reading →
Primary Method Feature: Kjeldahl (Part 1: Kjeldahl Foundations)
Why are we talking about Kjeldahl on the NIR blog? This blog has made many mentions to the fact that near-infrared (NIR) spectroscopy is a secondary method. That means that NIR doesn't measure things like moisture, fat, protein, ash, %-polymerization or anything else directly. Instead, we use an acceptable primary method, like Kjeldahl, to produce... Continue Reading →
BUCHI and CEMSI: Partners in Integration
A rapidly rising consumer food company needed NIR technology to quickly test final product quality in their pilot plant R&D facility. The R&D team wanted an NIR to provide non-contact, process control measurements of food products passing rapidly by on a conveyor belt. However, being a pilot facility, they desired adjustable and temporary mounting approaches... Continue Reading →
Fall is Doughnut Season (add an NIR for the perfect Baker’s Dozen)
In the northeast, fall ushers in crisp, cool air, vibrantly colored leaves... and doughnut season. Perfectly glazed Krispy Kreme Doughnuts Whether you're getting your round, deep-fried cake from roadside from the Pennsylvania Amish or from a commercial bakery, quality is key. Krispy Kreme has been serving up delicious doughnuts for generations in the USA and... Continue Reading →
Finding the right fit: FT vs Dispersive NIR
The longest continuously running mountain biking series in the United States is hosted in southwest Pennsylvania. Every year, hundreds of locals show up to test themselves in the 5-race series and vie for the title of "local hero." Races each take place at different venues, and each venue has a signature "wrench" to throw at... Continue Reading →
NIR vs. Raman: Spectroscopy Showdown
Raman and near-infrared (NIR) spectroscopy are complementary methods, both probing vibrational transitions in molecules. In general, the strong bands in the (N)IR spectrum of a compound correspond to weak bands in the Raman and vice versa. This blog will look at some of the differences between Raman (light-scattering) and NIR (light absorption) methods. Here just... Continue Reading →
Things are really getting cheesy at BUCHI.
When prodded, I suppose many at BUCHI would agree that some of the cheesiest members of the team belong to the NIR group. Maybe that's something to be proud of! More cheese, please! Cheese is delicious, after all. With a global market of around $100 Billion USD, I think there is a general agreement on... Continue Reading →
Quality is Going to the Dogs.
Don't worry, it's a good thing. This week, BUCHI Product and Application Specialists mingled with pet food suppliers and manufacturers at the Petfood Forum 2019 in Kansas City, MO. Some key topics on deck for event speakers include nutrition, labeling, product development, safety and manufacturing. Did you know BUCHI has its paws in the formulation,... Continue Reading →
NIR: a Spring-y subject
Winter felt brutal and eternal, as it always does for someone who doesn't ski or care for hot chocolate, I suppose. What a relief it is to see signs of Spring emerging from my brownish-colored yard and hear birds chirping outside once again. Did you know NIR is quite Spring-y as well. This blog will... Continue Reading →
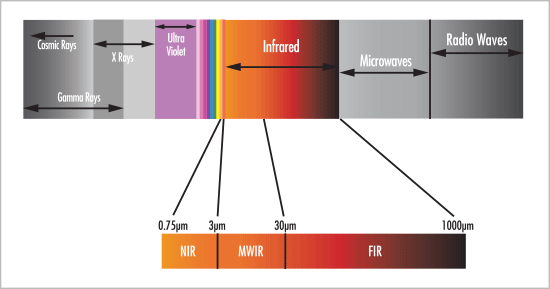
Near vs. Mid-IR: pick your poison
Is there a simple answer? Of course not! When it comes to the debate regarding which infrared spectroscopy reigns superior, near-infrared (NIR) or mid-infrared (IR), the answer should be a reflect the merits of the technology in light of the application of interest. It's like asking whether a knife is better than a spoon. Well,... Continue Reading →
Champion saves the day: Volume 2 Production
In-process and at-line NIR for production
Be a Food Analysis Champion!
Save time with efficient incoming goods inspection
Best Practices: Sample Planning for Quantitative NIR Methods
The focus of this post is NIR project and sample planning, a critical step in the NIR method development process that often gets rushed through in the eagerness to have our NIR instruments pump out measurement results. Putting some extra effort into sample planning could pay off big dividends in terms of two things I... Continue Reading →
Feed Manufacturing: Profit with In-line NIR Process Control
Is moisture content in feed synonymous with profit margin? Talk to a feed producer and you're likely to see a head nodding in agreement. How valuable would it be to have a continuous read-out of the moisture content of your feed at points along the process where you can still adjust your process to reach... Continue Reading →
Entering the Blogosphere
Why are we here?
Putting organic waste to use! How Near Infrared Spectroscopy supports recycling!
Hello, it’s Dr. Rukundo! For our first blog of 2026, we’re turning our attention to something both environmentally crucial and full of untapped potential: the recycling of organic waste. You’ve probably heard the saying, “One man’s trash is another man’s treasure.” When it comes to food waste, that statement holds truer than ever. Across the... Continue Reading →
NIR vs. Extraction for Fat Determination
Fat is a critical parameter across the food, beverage and feed industries. However, the best solution for obtaining fat varies across the production cycle. In this blog, we propose extraction and near-infrared (NIR) spectroscopy methods for fat determination, with arguments for which technology is the best fit based on factors such as: Application scopeVariation in... Continue Reading →
Considerations for Qualitative NIR Method Development
Goal-setting When we start to think about method development so many things come to mind. It’s a great idea to clearly define the goals of a project so that we can plan efforts and resources in such a way as to meet those goals. So, what should be our expectation, or goals, when we use... Continue Reading →